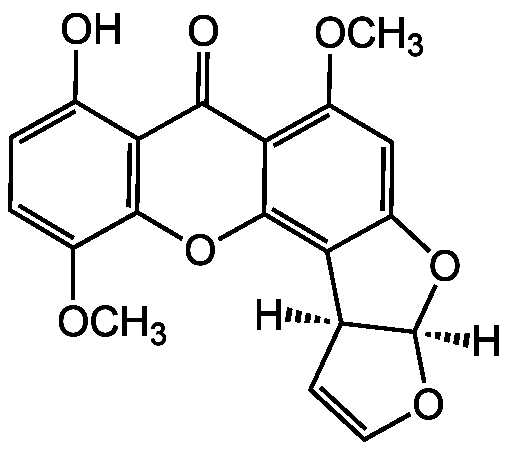
5-Methoxysterigmatocystin
Product Code:
BVT-0416
BVT-0416
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short term: +4°C. Long term: -20°C
Short term: +4°C. Long term: -20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0416-M001 | 1 mg | £111.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges to UK mainland customers, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
11-Methoxysterigmatocystin
Appearance:
Pale yellow powder.
CAS:
22897-08-1
Class:
6.1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS06,GHS08
Handling Advice:
Protect from light.
Hazards:
H301, H351
InChi:
InChI=1S/C19H14O7/c1-22-10-4-3-9(20)14-16(21)15-11(23-2)7-12-13(18(15)26-17(10)14)8-5-6-24-19(8)25-12/h3-8,19-20H,1-2H3/t8-,19+/m0/s1
InChiKey:
VVRUNWFPOWIBDY-WPCRTTGESA-N
Long Description:
Chemical. CAS: 22897-08-1. Formula: C19H14O7. MW: 354.3. Isolated from Aspergillus sp. (strain WDMH51). Mycotoxin. Derivative of sterigmatocystin. Carcinogenic activity. Anticancer compound. Cytotoxic and genotoxic.
MDL:
MFCD01725647
Molecular Formula:
C19H14O7
Molecular Weight:
354.3
Package Type:
Plastic Vial
PG:
III
Precautions:
P201, P281, P301, P310, P405
Product Description:
Mycotoxin. Derivative of sterigmatocystin. Carcinogenic activity. Anticancer compound. Cytotoxic and genotoxic.
Purity:
>98% (HPLC; NMR)
Signal Word:
Danger
SMILES:
[H][C@]12OC=C[C@@]1([H])C1=C3OC4=C(C(O)=CC=C4OC)C(=O)C3=C(OC)C=C1O2
Solubility Chemicals:
Soluble in chloroform and DMSO.
Source / Host:
Isolated from Aspergillus sp. (strain WDMH51).
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
5-Methoxysterigmatocystin, a metabolite from a mutant strain of Aspergillus vericolor: J. S. E. Holker & S. A. Kagal; Chem. Commun. 1968, 1574 (1968) | Fermentation, isolation, and antitumor activity of sterigmatocystins: W.T. Bradner, et al.; Antimicrob. Agents Chemother. 8, 159 (1975) | Preparation and antitumor activities of some derivatives of 5-methoxysterigmatocystin: J.M. Essery, et al.; J. Med. Chem. 19, 1339 (1976) | 1H and 13C NMR assignments for two anthraquinones and two xanthones from the mangrove fungus ZSUH-36: C. Shao, et al.; Magn. Reson. Chem. 45, 434 (2007) | Sterigmatocystins from deep-sea-derived fungus Aspergillus versicolor: C. Shengxin, et al.; J. Antibiot. 64, 193 (2011) | Cytotoxicity and genotoxicity of versicolorins and 5-methoxysterigmatocystin in A549 cells: D. Jksic et al.; Arch. Toxicol. 86, 1583 (2012) | Secondary Metabolites of the Marine Fungus Aspergillus versicolor SCSIO 05772: X. Song, et al.; Chem. Nat. Compd. 53, 354 (2017)