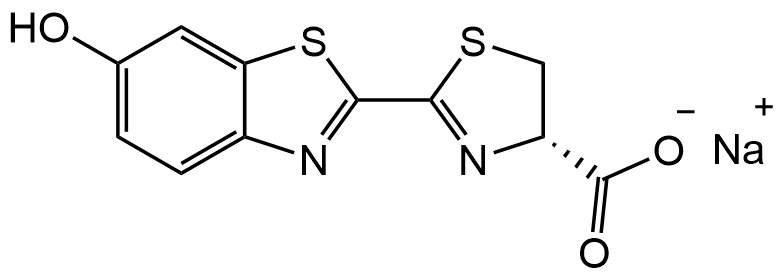
D-Luciferin sodium salt
Product Code:
CDX-L0008
CDX-L0008
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short term: +4°C. Long term: -20°C
Short term: +4°C. Long term: -20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-L0008-M050 | 50 mg | £46.00 |
Quantity:
| CDX-L0008-M250 | 250 mg | £151.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges to UK mainland customers, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Appearance:
Faint to dark yellow powder.
CAS:
103404-75-7
Description:
D-Luciferin sodium salt is a water-soluble format of D-Luciferin. D-Luciferin is the most popular and versatile bioluminescent substrate. The firefly luciferase/luciferin bioluminescent system is found in the firefly (Photinus pyralis) and several other beetles. Firefly luciferase produces light by the ATP-dependent oxidation of D-Luciferin. The 560nm chemiluminescence from this reaction peaks within seconds, with light output that is proportional to luciferase activity when D-Luciferin and ATP are present in excess. Luciferase is encoded by the luc gene, which is widely used as a reporter gene in a variety of cells. Because of the intrinsic low background of chemiluminescence technique, detection of the luc gene expression can be made at very low level. In addition, luciferin/luciferase has been used to measure 10-15 molar quantity of ATP. D-luciferin and luciferase can be used to assess ATP availability in cellular or biochemical assays. Firefly luciferase has long been conjugated to antibodies and used as a label in immunoassays using D-Luciferin as the substrate for detection. Compared to HRP and alkaline phosphatase, luciferase is less tolerant to chemical modifications. One particular advantage to the enzyme is that there is low endogenous luciferase activity in mammalian tissues besides its high sensitivity. Another important use of luciferase is in the area of hygiene monitoring. The luciferase/luciferin system can be used to detect contamination because ATP, present in all living organisms, is required to produce luminescence. The main application for this type of ATP bioluminescence is quality assurance by testing surfacesin food processing plants to determine whether or not there iscontamination of eitherequipment or products.
EClass:
32160000
Form:
solid
Handling Advice:
Protect from light and moisture. Very light-sensitive.
InChi:
InChI=1S/C11H8N2O3S2.Na/c14-5-1-2-6-8(3-5)18-10(12-6)9-13-7(4-17-9)11(15)16;/h1-3,7,14H,4H2,(H,15,16);/t7-;/m1./s1
InChiKey:
LILQLBIQROYWIA-OGFXRTJISA-M
MDL:
MFCD00044938
Molecular Formula:
C11H7N2NaO3S2
Molecular Weight:
302.3
Package Type:
Vial
Purity:
>98% (HPLC)
SMILES:
O=C([O-])[C@H]1CSC(C(S2)=NC3=C2C=C(O)C=C3)=N1.[Na+]
Solubility Chemicals:
Soluble in water, PBS (pH7.2), DMSO or DMF (all 10 mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) A.R. Brasier, et al.; Biotechniques 7, 1116 (1989) | (2) W.J. Simpson, et al.; Lett. Appl. Microbiol. 1, 208 (1990) | (3) N. Lembert, et al.; Biochem. J. 317, 273 (1996) | (4) D.R. Lasko & D.I. Wang; Biotechnol. Bioeng. 52, 364 (1996) | (5) F. Brau, et al.; BBRC 270, 247 (2000) | (6) W. Wang & W.S. El-Deiry; Cancer Biol. Ther. 2, 196 (2003) | (7) K. Niwa, et al.; FEBS Lett. 580, 5283 (2006) | (8) Y. Inoue, et al.; Eur. J. Nucl. Med. Mol. Imaging 36, 771 (2009) | (9) S.T. Smale; Cold Spring Harb. Protoc. 2010, pdb.prot5421 (2010) | (10) Y.Q. Sun, et al.; Angew. Chem. Int. Ed. Engl. 51, 8428 (2012) | (11) S.T. Adams & S.C. Miller; Curr. Opin. Chem. Biol. 21, 112 (2014) (Review)