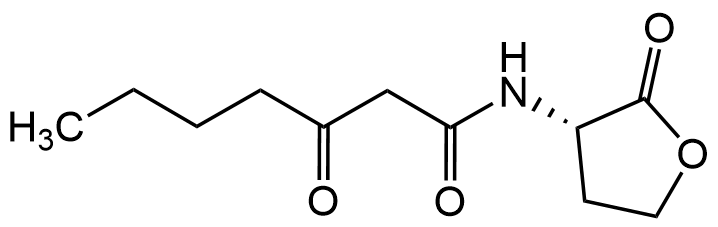
N-(3-Oxoheptanoyl)-L-homoserine lactone
Product Code:
CDX-O0138
CDX-O0138
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short term: +4°C. Long term: -20°C
Short term: +4°C. Long term: -20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-O0138-M010 | 10 mg | £79.00 |
| CDX-O0138-M050 | 50 mg | £278.00 |
Prices exclude any Taxes / VAT