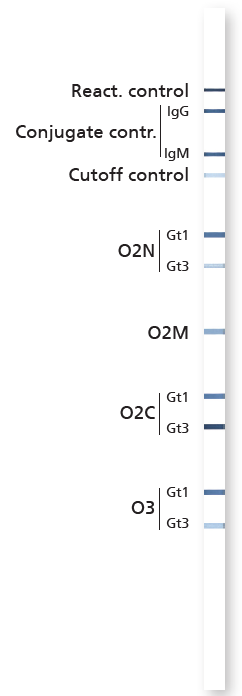
Mikrogen Diagnostik recomLine HEV IgG/IgM Lateral Strip Test Kit
Product Code:
LEI-5072
LEI-5072
Host Type:
Virus
Virus
Regulatory Status:
RUO
RUO
Application:
Lateral-Flow Immunochromatographic Assay
Lateral-Flow Immunochromatographic Assay
Shipping:
2-8°C
2-8°C
Storage:
Store at 2-8°C until expiration on packaging.
Store at 2-8°C until expiration on packaging.
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| LEI-5072-20tests | 20 Tests | £825.00 |
Prices exclude any Taxes / VAT