After being degraded by specific enzymes, the triple helical collagen molecule becomes thermally unstable and spontaneously unfolds at body temperature, leaving denatured collagen fragments covalently crosslinked within the partially degraded matrix.[1,2] Such denatured collagen is a hallmark indicator of inflammation and tissue damage under many pathological conditions, as well as programmed tissue remodeling during physiological and developmental events. 3Helix’s Collagen Hybridizing Peptide (CHP) is the first and only agent that specifically targets this biomarker in histopathology.[1] This powerful tool may enable straightforward applications in disease diagnosis, staging, as well as facile evaluation of therapeutic candidates by histology. CHP is applicable to nearly all tissue types and a wide range of medical areas, such as myocardial infarction, arthritis, nephritis, and fibrosis.
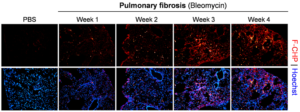
Pulmonary fibrosis. Representative fluorescence micrographs of the subpleural areas of lung cryosections obtained from mice dosed with bleomycin through minipumps for varying time periods versus control mice dosed with PBS for 1 week, and stained with F-CHP and Hoechst 33342. Highly localized, bright F-CHP signals revealed spotty distribution of damaged collagen appearing beginning 1 week after bleomycin treatment. The overall CHP signals increased as the disease progressed from week 1 to week 3 and persisted through week 4.[1]
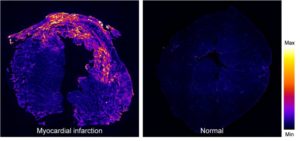
Myocardial infraction. A fluorescence image of a mouse heart at day 7 post myocardial infraction (left), stained by biotin-labeled B-CHP (detected with AlexaFluor647-streptavidin), in comparison to a normal heart (right). Intense signals resulting from CHP binding to the degraded and denatured collagen can be seen in the damaged myocardial tissue.[1]
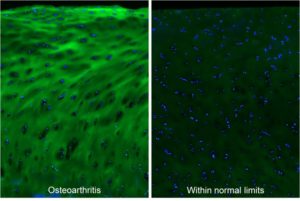
Osteoarthritis. Micrographs of articular cartilage tissue within the osteoarthritic or normal region from an osteoarthritis patient, stained by fluorescein-labeled F-CHP (green) and Hoechst 33342 (blue), showing high levels of degraded type II collagen in the degenerated cartilage.[1]
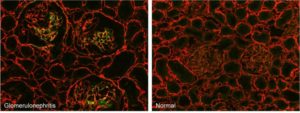
Glomerulonephritis. Micrographs of kidney cryosections from anti-Thy-1 nephritic and normal rats stained with fluorescent F-CHP (green) and an anti-collagen IV antibody (red). The F-CHP signal is confined to the damaged collagen in the inflamed glomeruli,[1] whereas the collagen IV antibody visualizes all collagen IV content uniformly distributed in the tissue.
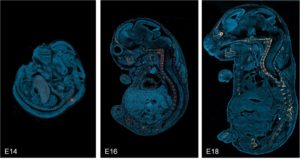
Bone development. Localization of CHP binding in sagittal cryosections of mouse embryos from day 14 to 18 post-coitum (E14-18) that were stained with fluorescein-labeled F-CHP (orange/yellow) and Hoechst 33342 (blue), clearly showing the collagen degradation detected by CHP was localized exclusively in the developing skeletal tissue during this endochondral ossification process,[1] wherein a pre-formed cartilage template is resorbed and replaced by mineralized bone.
This example demonstrates that CHP can be used in the research of developmental biology of the skeletal system; it may be particularly useful for studying genetic disorders or factors in skeletal development, and for characterizing animals with a broad spectrum of skeletal phenotypes (e.g., diversity outbred mice).
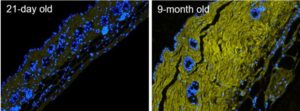
Skin aging. Fluorescence micrographs of formalin-fixed paraffin-embedded skin sections from 21-day old versus 9-month old mice, stained with Hoechst 33342 (blue) and biotin-labeled B-CHP (detected by AlexaFluor647-streptavidin, yellow), displaying significantly greater amount of degraded collagen in the dermis of the aged skin. Reprinted from ref [1] with permission. In addition to chronological skin aging, CHP may be used to detect collagen damage in photoaging, skin inflammation and infection, and autoimmune skin disorders, as well as to evaluate the effectiveness or safety of skin care products and ingredients.
Applicable organs and tissues:blood vessels, heart, lung, liver, kidney, muscles, gastrointestinal tract, genital system, breast, bone, joints, soft tissues, cartilage, ligament, tendon, oral cavity, embryo, eyes, skin
Potential disease and medical areas:atherosclerosis, giant cell arteritis, aneurysm, myocardial infarction, respiratory inflammation, pneumonia, asthma, pulmonary fibrosis, effects of air pollution, liver fibrosis, cirrhosis, glomerulonephritis, kidney fibrosis, inflammatory bowel disease, bladder inflammation, prostatitis, uterine fibroid, cervical cancer, mammary development, breast cancer, muscle disorders, muscular dystrophy, osteoporosis, bone fracture, intervertebral disc degeneration, spondylosis, osteoarthritis, rheumatoid arthritis, gout, lupus, scleroderma, orthopedics, sports medicine, rotator cuff tear/disease, tendinosis, whiplash, ligament tear, trauma, oral inflammation, periodontitis, oral mucositis, skeletal development, genetic connective tissue disorders, marfan syndrome, collagen mutations, osteogenesis imperfecta, cornea injury & infection, LASIK, keratoconus, fuchs’ dystrophy, glioblastoma, photoaging, skin cancer, melanoma, skin disorder, dermatitis, rheumatic skin diseases, cosmetic dermatology, diabetic ulcer, cancer invasion and metastasis, foreign body response to implants & transplants, angiogenesis, wound healing, ulcers, adhesion