Spatial biology is adding new dimensions to translational medicine.
Spatial biology is adding new dimensions to translational medicine. Advances in biomarker analysis have led to major breakthroughs in cancer research, translational medicine, therapy and diagnostics. Progress has been powered by rapid data generation using high throughput methods such as next-generation sequencing and proteomics based on mass spectrometry. With much of the low-hanging fruit already harvested, innovative therapies, such as immunotherapy, are offering new ways to combat cancer at the level of the individual patient. Biomarkers help to fine-tune this personalised, or precision, medicine in the form of companion diagnostics that guide the use of personalised treatment options, for example in breast cancer.
Progress now depends on going to the next level – mapping biomarkers in space and time to elucidate the evolving tumour microenvironment (TME). This is where spatial biology and spatial-omics (or spatial multiomics), which integrates and visualises transcriptomic and proteomic data, provide invaluable insights.
Spatial-omics – mapping the progress of cancer in 3D
The diverse individual cell types and cell states of tumours create a highly complex and dynamic TME that can only be fully understood by spatial analysis. Spatial-omics is now extending cancer research and diagnosis resolution from the tissue or organ to the cellular and subcellular level. Spatial phenotyping, based on techniques such as multiplex immunohistochemistry (IHC) or multiplex immunofluorescence (mIF), is proving to be particularly valuable and involves tissue imaging with single-cell resolution to visualise and quantitate biomarker expression to map the organisation of cells in the tissue and how they interact.
Combining spatial-omics data, such as gene expression data and protein co-detection data can add a new dimension of information – for example, patterns and associations formed by individual cells with specific phenotypes and functions that are not evident with other methodologies (cellular neighbourhoods), and posttranslational modification and subcellular localisation of proteins and their dysregulation in disease. Spatial transcriptomics and spatial phenotyping are also supporting the drive towards personalised (individualised) medicine.
Spatial phenotyping brings new insights and increases predictive power
There are many examples of how combining biomarkers can bring new insights into disease and therapeutic mechanisms and increase predictive power. Spatial phenotyping, based on multiplex IHC, for example, promises to bring this to a new level. For example, cancer immunotherapy is revolutionising cancer treatment by boosting the natural immune response and advances depend on a deep understanding of the TME. Multiplexed immunophenotyping can give new insights into this and is essential in identifying predictive biomarkers of response and gaining insights into therapeutic mechanisms of action.
One example that illustrates the predictive power of spatial phenotyping is the meta-analysis by Steve Lu and coworkers, based at Johns Hopkins Medical Institutions, Baltimore, USA aimed at determining the value of this approach in predicting patient response to anti-PD-1/PD-L1 (programmed cell death ligand 1) therapy. Their analysis was built on reports on the diagnostic accuracy of tumour mutational burden (TMB), gene expression profiling (GEP), and spatial phenotyping using multiplex immunohistochemistry/immunofluorescence (mIHC/IF). The meta-analysis showed that while TMB, PD-L1 IHC, and GEP alone had comparable areas under the curve (AUC) in predicting response to treatment, mIHC/IF and multimodality biomarker strategies were better at predicting response (Figure 1.).
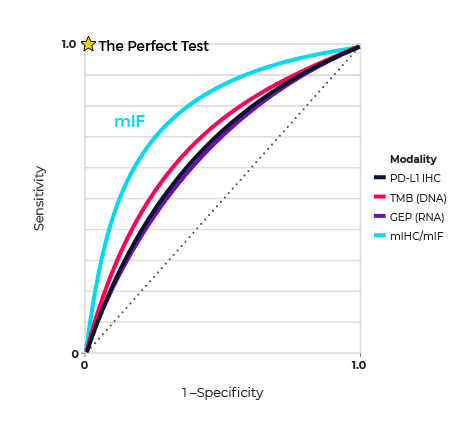
The researchers concluded that “The relative success of mIHC/IF in predicting patient response also provides insight into the spatial importance of tumour-immune interactions and the contribution of protein marker co-expression”. What was striking was that this success was achieved with an average of only 2–3 biomarkers, which suggests that exploiting the large multiplexing capacity of mIHC/mIF has the potential to improve diagnostic accuracy even more.
Staying ahead with spatial phenotyping
Spatial phenotyping certainly highlights the wealth of valuable data that can be extracted from a tissue slide. The power of this approach is so great that it has been suggested by Matt Humphries, scientific lead for Tissue Hybridisation and Digital Pathology at Queen’s University Belfast, Northern Ireland, that labs that fail to include spatial phenotyping in their research run the risk of being left behind.
Spatial phenotyping using multiplex IHC
Several multiplex imaging techniques have been developed to generate spatial multi-biomarker data from biopsies. One approach is the automated multiplex system PhenoCycler-Fusion (formerly CODEX®; Akoya Technologies; https://www.leinco.com/codex_technology/) that uses DNA barcode technology comprised of unique oligonucleotide sequences conjugated to an antibody. An initial step involving staining tissue with up to 100 uniquely barcoded antibodies is followed by cycles of visualising three antibodies at a time with reporter dyes linked to complimentary barcodes. This enables the visualisation and quantification of dozens of biomarkers in a single tissue sample while maintaining cellular and sub-cellular detail.
Benefits of the system include:
- A comprehensive end-to-end solution containing the fluidics platform, assay reagents, and bioinformatics
- Benchtop footprint that integrates with an existing fluorescence microscope
- The system is scalable and capable of imaging up to 100 biomarkers per run
- Preserves the sample for region-of-interest (ROI) analysis and haematoxylin and eosin (H&E) staining
- Fresh frozen (FF) and formalin-fixed, paraffin-embedded (FFPE) tissue compatible The system provides an accurate picture of cell neighbourhoods and interactions in the tissue microenvironment and has been used in many studies involving spatial phenotyping in cancer research (for example, refs. 1 & 6).
Reliable and reproducible spatial phenotyping depends on high-quality antibodies
Confidence in data is critical and the reproducibility of analytical methods has become a major issue. The reproducibility of multiplex immunofluorescence (mIF) for spatial phenotyping depends on many factors, including antibody selection, antibody optimisation and validation, panel design, staining optimisation and validation, analysis strategies, and correct data generation. One of the most important steps in ensuring reproducibility is the evaluation of the performance of antibodies in IHC. The International Working Group for Antibody Validation, for example, proposed five approaches for antibody validation in general:
- genetic
- orthogonal
- independent antibody strategies
- expression of tagged proteins
- immunocapture followed by mass spectrometry
At least one of the five approaches should be used to validate an antibody for a particular application. The antibodies used in mIF should therefore be validated for the application and purified to ensure a high signal/noise ratio to ensure the fidelity of the analysis.
Conclusions
Spatial phenotyping is already delivering new insights into how cancer develops and progresses, providing ways to increase diagnostic accuracy, and guiding the development of innovative therapies such as immunotherapy. Confidence in results builds on many factors, including high-quality antibodies that enable the generation of reliable and reproducible data.
References
- Phillips, D et al. Highly Multiplexed Phenotyping of Immunoregulatory Proteins in the Tumor Microenvironment by CODEX Tissue Imaging. Front. Immunol., 19 May 2021 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8170307/
- Lu S, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol. 2019 Aug 1;5(8):1195-1204. doi: 10.1001/jamaoncol.2019.1549. PMID: 31318407; PMCID: PMC6646995.
- Blog post: Five publications that left their mark on spatial biology. Akoya Biosciences. https://www.akoyabio.com/blog/
- Staying Ahead of the Rest with Spatial Phenotyping. The Pathologist. 04/07/2021 https://thepathologist.com/diagnostics/staying-ahead-of-the-rest-with-spatial-phenotyping
- Allam, M. et al. Multiplex bioimaging of single-cell spatial profiles for precision cancer diagnostics and therapeutics. Precision Oncology volume 4, Article number: 11 (2020) https://www.nature.com/articles/s41698-020-0114-1
- Schürch, CM et al Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front Cell 182 (5), 3 September 2020, Pages 1341-1359.e19 https://www.sciencedirect.com/science/article/pii/S0092867420308709
- Baker M. 1,500 scientists lift the lid on reproducibility. Nature. 2016 May 26;533(7604):452-4. doi: 10.1038/533452a. https://www.nature.com/news/1-500-scientists-lift-the-lid-on-reproducibility-1.19970
- Freedman LP, et al. The Economics of Reproducibility in Preclinical Research. PLoS Biol. 2015 Jun 9;13(6):e1002165. doi: 10.1371/journal.pbio.1002165. eCollection 2015 Jun. http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1002165#sec010
- Laberiano-Fernández, C. et al. Best practices for technical reproducibility assessment of multiplex immunofluorescence. Front Mol Biosci. 2021 Aug 31;8:660202. doi: 10.3389/fmolb.2021.660202. eCollection 2021. PMID: 34532339
- Uhlén M, et al. A proposal for validation of antibodies. Nat Methods. 2016 Oct;13(10):823-7. doi: 10.1038/nmeth.3995. Epub 2016 Sep 5. PMID: 27595404.
Originally posted by Leinco Technologies Inc. on: https://www.leinco.com/what-is-spatial-biology/
Caltag Medsystems is the distributor of Leinco Technologies’ products in the UK and Ireland. If you have any questions about these products, please contact us.
