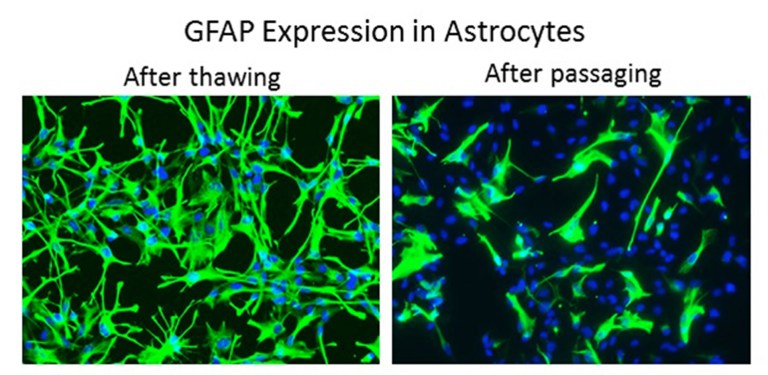
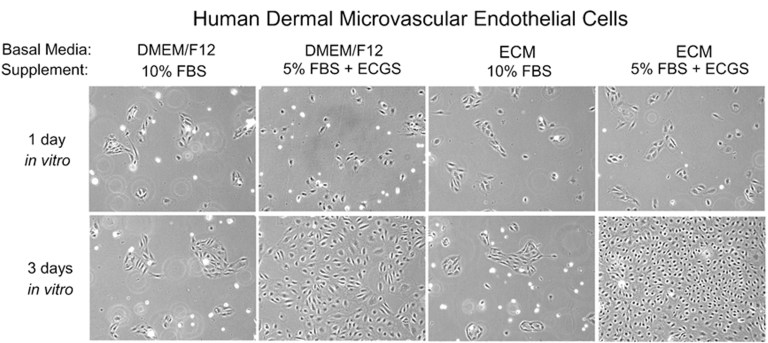
When working with primary cells, it is important to remember that they are not cell lines and should be treated with care. At ScienCell, they specialise in primary cell culture and are very familiar with the common problems researchers encounter when culturing them. They have compiled a list of 13 of the most common problems that researchers encounter when culturing primary cells.
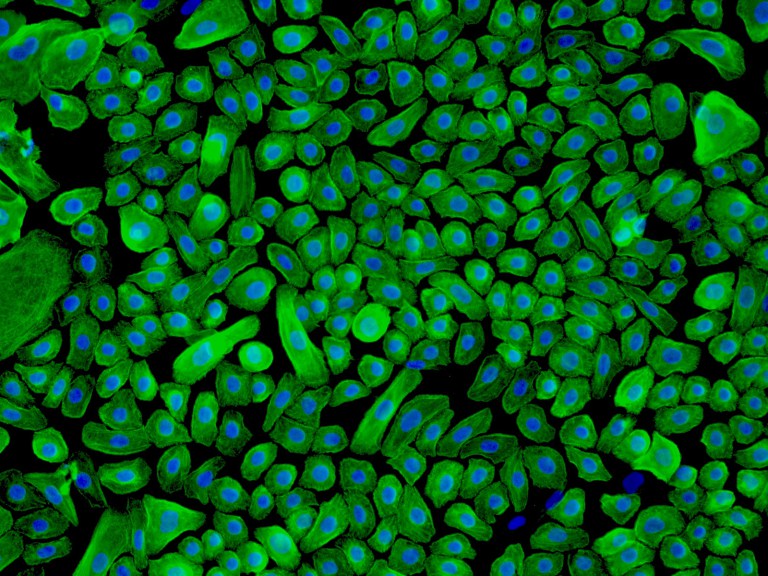 Human Epidermal Keratinocytes-neonatal (HEK-n) – Immunostaining for CK-18, 200x.
Human Epidermal Keratinocytes-neonatal (HEK-n) – Immunostaining for CK-18, 200x.
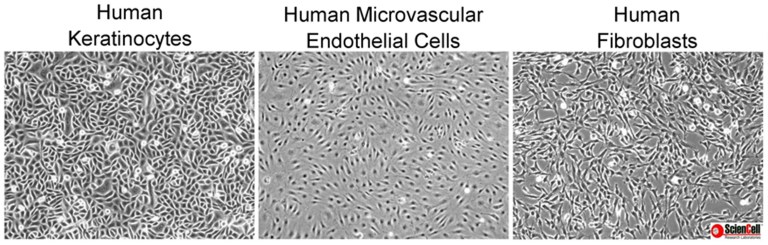
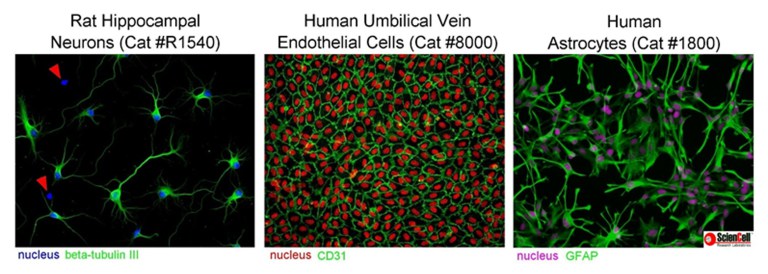
Mistake #1: Being unfamiliar with the primary cell types being cultured.
Correction #1: It is very important to know the morphology of primary cells and to be aware of the morphology of potentially contaminating cells.
Mistake #2: Primary cells are 100% pure.
Correction #2: Primary cells are rarely 100% pure so it is essential to pay close attention to cell morphology and to not allow cells to overgrow.
Mistake #3: Thawing a vial of primary cells in a water bath for longer than necessary.
Correction #3: Primary cells are very sensitive to the thawing process so it is important that the vial be placed in a 37°C water bath, held and rotated gently until the contents are just thawed. Remove the vial from the water bath promptly and transfer it into a sterile hood. Make sure your culture vessel is ready before thawing so the cells can immediately be seeded and placed in the incubator.
Mistake #4: Centrifuging primary cells directly after thawing a vial.
Correction #4: ScienCell does not recommend centrifuging the cells after thawing because the centrifugation procedure is more harmful than the minimal DMSO residue. Remember to change medium the following day after reviving primary cells to remove any residual DMSO.
Mistake #5: Primary cells need to be triturated vigorously with a pipette to resuspend cells.
Correction #5: Primary cells are very sensitive, especially after thawing, and should only be gently resuspended using a pipette. The purpose of the resuspension is to ensure that the cells are plated evenly. Any additional shear forces can damage the primary cells.
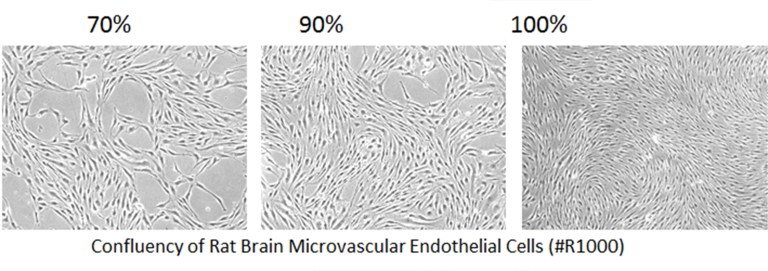
Mistake #6: Allowing primary cells to become too confluent.
Correction #6: Primary cells can become senescent when grown to 100% confluence. Remember that primary cells are not 100% pure, so it is important to minimise the growth of contaminating cells. ScienCell recommends subculturing primary cells when they are 90-95% confluent.
Mistake #7: Subculturing primary cells will improve the health and morphology of them.
Corrections #7: Actually, trypsin can be harmful to cells and passaging primary cells too early or too frequently is not beneficial to them.
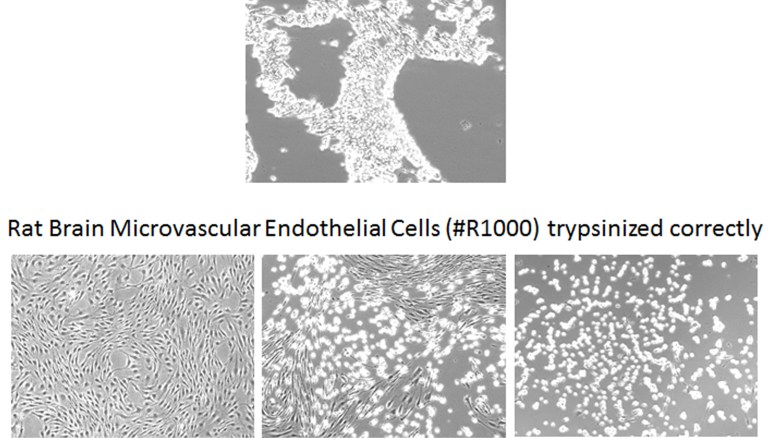
Mistake #8: Over-trypsinisation when passaging primary cells.
Correction #8: When passaging cells, use low concentrations of trypsin and monitor cells closely under the microscope. Also, remember to completely neutralise the cells after trypsinisation because any active trypsin will damage the cells. ScienCell specifically recommends a trypsin neutralisation solution (Cat #0113) specially formulated for primary cells, but 10% serum or soybean trypsin inhibitor (Cat #0173) can also be used.
Mistake #9: A population doubling and a passage are interchangeable.
Correction #9: A population doubling and a passage are actually very different. A population doubling is a two-fold increase in the total number of cells in a culture. Passage number is the number of times that a cell population has been removed from the culture vessel and undergone a subculture. In other words, passage number is dependent on the user, whereas, population doubling is dependent on the rate of cell growth.
Mistake #10: Using primary cells for experiments past the expected population doubling.
Correction #10: ScienCell recommends using primary cells as early as possible to limit functional changes. As primary cells proliferate and adapt to the 2D cell culture environment, protein expression can be altered over time. Even if cells continue to proliferate they may not be appropriate for experiments.
Mistake #11: Primary cells can easily be refrozen.
Correction #11: Normally, ScienCell does not recommend refreezing primary cells as this can promote a senescent phenotype and/or cause functional changes. Primary cells are extremely sensitive and refreezing may result in cell death or damage.
Mistake #12: Primary cells can proliferate indefinitely.
Correction #12: Unlike cell lines, primary cells have a limited expansion capacity. ScienCell recommends using primary cells as early as possible for experiments to prevent genetic drift. In addition, when working with a difficult cell type monitor cell morphology closely for contaminating cells as they can take over the culture. At ScienCell, they indicate the expected population doubling for each cell type on their primary cell product sheets.
Mistake #13: Using a classical medium for culturing primary cells rather than using a specialty medium.
Correction #13: Classical medium was developed to support the growth of cell lines, while specialty medium is optimised for each primary cell type and designed to support the more complex nutritional requirements of primary cells. Specialty media are often designed to be used with little or no serum, so the media is supplemented with the beneficial components in serum (like growth factors, lipids, and hormones) to compensate. Serum contains growth inhibitory factors, so using low serum or serum-free media reduces the negative effects of these components. At ScienCell, they have developed over 70 different specialty media designed to support many unique primary cell types, including endothelial cells, astrocytes, pericytes, neurons, smooth muscle cells, keratinocytes, epithelial cells, and microglia.
Overall, remember to treat primary cells with care and to be familiar with the cells you are culturing. Use cells early for experiments to prevent genetic drift and functional changes. ScienCell recommends following these technical tips for successful primary cell culture.
For further information about ScienCell please click here.
Originally posted on https://sciencellonline.com/blog/13-technical-tips-for-successful-primary-cell-culture/
Caltag Medsystems is the distributor of Sciencell products in the UK and Ireland. If you have any questions about these products, please contact us.