The promise for cell and gene therapies (CGTs) to revolutionise cancer therapy is immense, yet unlocking their full potential to treat more diseases and improve patient access will depend on the selection of donors who can provide high-quality cells and tissues. By addressing the challenges of program-specific donor selection and ensuring that the apheresis material meets strict quality standards suitable for downstream Good Manufacturing Practice (GMP) manufacturing of CGTs, developers can streamline the path towards clinical translation and regulatory approval.
One of the main challenges for allogeneic CGT is donor variability, which can make building robust manufacturing processes difficult. This variability translates to variability in the final CGT product, which can significantly impact therapeutic efficacy, cost, dosage, and more. Donor characteristics such as T cell content, activation potential, expansion, and transduction efficiency all contribute to the production of successful CGT products. Careful donor selection can minimise variability and provide opportunities to implement process controls, which is why many allogeneic programs have specific donor attribute requirements.
The donor selection process can be lengthy, from several weeks to many months, depending on the specific donor criteria required. The need to recall potential donors for whole blood screening and extensive characterisation can cause severe bottlenecks, leading to delays in the selection process. Moreover, traditional methods of donor selection often fall short in providing a comprehensive assessment of donor suitability, resulting in unwanted variability that can impact the efficacy and therapeutic potential of the final CGT product and in turn, impact clinical responses.
SEAMLESSLY NAVIGATE THE DONOR SELECTION PROCESS
AllCells has recently introduced the Pre-Characterised Donor Selection Program designed to expedite screening, selecting, and collecting from eligible donors (Figure 1) for downstream clinical manufacturing. Many CGT programs have specific donor requirements, narrowing the pool of potential donors that meet pre-determined criteria, which can result in a lengthy donor selection process. AllCell’s program provides immediate access to comprehensive donor characterisation data to effectively navigate the selection process and choose the most suitable candidates.
How does the program work?
Clients initially approach AllCells’ supplier, Caltag Medsystems, with specific criteria for donors that align with their program requirements. In collaboration with AllCells’ team of experts, clients gain access to a wealth of extensive characterisation data from eligible donors.
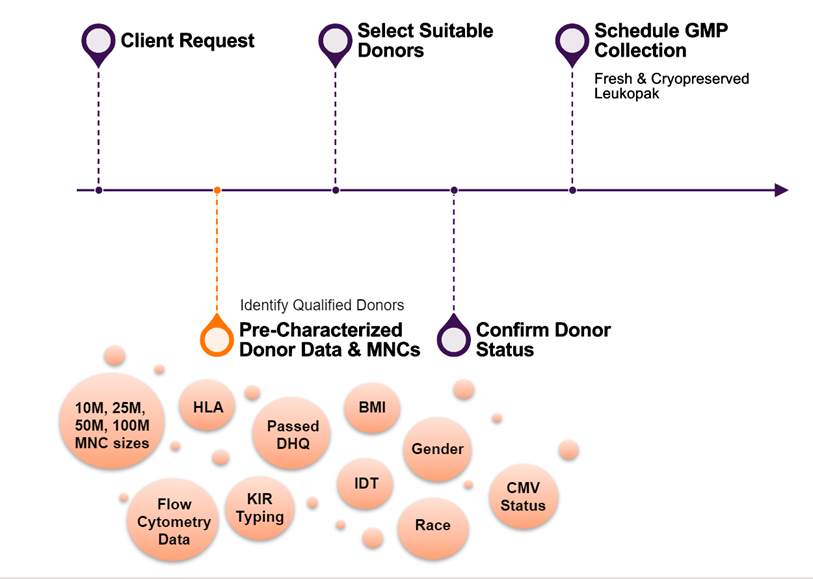
These are individuals whom AllCells knows in real-time – through their live donor dashboard – meet specific clinical eligibility criteria, such as age, BMI, medical history, and last date tested for viral testing in accordance with 21 CFR 1271 regulations. Histocompatibility analysis including Human Leukocyte Antigen (HLA) typing and KIR (killer cell immunoglobulin-like receptor) profiling for each donor is available in addition to 9-color flow panel immunophenotyping analysis performed on cryopreserved mononuclear cells (MNCs) collected from these eligible donors (Table 1).
| CELL SUBPOPULATION | MARKERS |
|---|---|
| Leukocytes | CD45+ |
| Monocytes | CD45+CD14+ |
| Classical Monocytes | CD45+CD14+CD16- |
| Intermediate Monocytes | CD45+CD14+CD16+ |
| Non-Classical Monocytes | CD45+CD14dimCD16+ |
| B Cells | CD45+CD14-CD19+ |
| NK Cells | CD45+CD14-CD19-CD3-CD56+ |
| CD16+ NK cells | CD45+CD14-CD19-CD3-CD56+CD16+ |
| CD16- NK cells | CD45+CD14-CD19-CD3-CD56+CD16- |
| CD3 T Cells | CD45+CD14-CD19-CD3-CD56- |
| CD8 T Cells | CD45+CD14-CD19-CD3+CD56-CD8+ |
| CD4 T Cells | CD45+CD14-CD19-CD3+CD56-CD4+ |
| NKT Cells | CD45+CD14-CD19-CD3+CD56+ |
| Ratio CD4/CD8 | See above |
Once the list of suitable candidates is compiled from AllCells’ database, their donor management team coordinates the scheduling of these donors for collections of GMP fresh or cryopreserved leukopak products collected under Good Tissue Practice (GTP). This comprehensive and streamlined approach enables AllCells’ clients to save valuable time and obtain high-quality starting cellular materials to accelerate the advancement of their therapies. This framework is particularly beneficial for programs that require screening a large number of donors to improve selection efficiency, allowing for a more seamless process from candidate selection to materials collection.
Customisable characterisation
AllCells understands that some clients may have specific characterisation requirements that go beyond what is covered by the Pre-Characterised Donor Selection Program. They offer custom pre-screening solutions using cryopreserved MNCs, whole blood or leukopaks to accommodate clients seeking donors with specific genetic markers, phenotypic characteristics, or other desired attributes. Leveraging AllCells’ robust analytical capabilities, they can obtain the necessary data needed to make informed decisions and progress programs successfully.
CGT MANUFACTURING REQUIRES HIGH-QUALITY STARTING MATERIALS
After the identification of suitable donors using the Pre-Characterised Donor Selection Program, the donors are scheduled for leukopak collections. AllCells offers both fresh and cryopreserved GMP leukopaks collected using the continuous flow Spectra Optia® Apheresis System directly into ACD-A anticoagulant following a standardised protocol at our FDA-registered, AABB-compliant collection facilities.
AllCells’ processing lab is located adjacent to the collection site, enabling immediate processing for cryopreservation in CryoStor10 within ISO 7-certified clean rooms using a proprietary Master Batch Record (MBR) optimised to preserve cellular quality and functionality. Cryopreservation at the peak of viability and functionality preserves cell phenotypes and allows cells to be stored for use later with minimal loss in viability and potency. With the ability to store/stockpile cryopreserved leukopaks until needed, researchers gain simplified logistics, timeline flexibility, and improved deliverability.
In an industry where standardisation for starting raw cellular materials is lacking, working with a reliable supplier with proven expertise, optimised protocols, and a robust regulatory compliance framework for GMP products is invaluable—not all suppliers can provide materials at this level of compliance. This partnership can significantly accelerate workflows, enhance speed-to-market, and provide supply chain security as a therapeutic program matures.
Built-in risk mitigation ensures deliverability
Donors are human beings, and AllCells understands that there are factors that can affect their eligibility and/or ability to come in for scheduled collections. That’s why they have built-in risk mitigation strategies to build reliability and minimise the occurrence of donor deferrals that disrupt the supply chain to ensure product deliverability.
- Active donor monitoring: viral testing at two-time points (≤30D and ≤7D) prior to collection to ensure donor eligibility
- Parallel Donor Program that qualifies an additional backup clinical donor in case of deferral
AllCells’ team can provide comprehensive support and a streamlined workflow for allogeneic cell sourcing from donor identification through coordination of cell collection and delivery of high-quality GMP products. They also help their clients strike the right balance between the donor attribute profile and long-term sustainability to avoid challenges from overly narrow criteria that can restrict donor selection.
As a trusted leader in the field, AllCells has the expertise and innovative solutions to help developers overcome the challenge of donor selection for GMP apheresis products used in CGT programs. AllCells offers a holistic approach to donor selection. Their industry-driven donor ecosystem, scalable apheresis network, advanced screening and characterisation techniques beyond the basic clinical qualification criteria, like immune cell phenotyping, and purpose-built management and risk mitigation strategies collectively contribute to finding the right donors for every CGT program.
Originally posted by AllCells on https://allcells.com/blog-overcome-gmp-donor-selection-challenges/
Caltag Medsystems is the distributor of AllCells products in the UK and Ireland. If you have any questions about these products, please contact us.
