Bispecific Antibody drugs have gained considerable popularity since the approval of blinatumomab. Currently, six bsAb drugs have been approved by the US FDA / EMA and over 180 are currently in clinical trials. BsAb simultaneously recognises different antigens or epitopes, resulting in a diverse range of therapeutic mechanisms. With a ‘two-target’ functionality that expands their use for therapeutic applications considerably, bsAb drugs have emerged as a promising class of therapeutics for a range of diseases, including cancer, autoimmune disorders, and infectious diseases.
The vast number of therapeutic applications due to their functionality results in a vast diversity within the bsAb family, including more than 100 different combinations of antigen-binding moieties and (homo/hetero) dimerization modules, classified based on their format (fragment-based, symmetric, and asymmetric) and their valency (number of binding sites, generally 1 + 1, 1 + 2, or 2 + 2)
Among the bsAb programs currently under development, the combination of CD3 and tumour surface targets are the most popular target pairs. Other popular targets are CD3, HER2, PD-1, PD-L1, EGFR, CTLA-4, etc., as well as immune targets of PD-1, PD-L1, BCMA, CD47, CTLA-4, LAG-3, 4 -1BB. Additionally, with the approval of the first immune checkpoint bsAb (Cadolinimab, approved 2022), and new mechanisms for improving efficacy like the development of hetero-dimer bispecific molecules, several additional possibilities of target pairs have emerged.
Currently, the MOAs of bsAbs in the treatment of lung cancer mainly include the following three categories: bridging immune cells with tumour cells for redirected cytotoxicity, simultaneous blockade of two signalling pathways to inhibit tumour growth, and targeting dual immunomodulatory molecules to promote immune responses.
Several bsAbs in clinical development are designed to redirect T cells to tumour cells. This process is accompanied by the formation of a transient cytolytic synapse between the T cell and the targeted tumour cell. The subsequent activation and proliferation of T cells leads to tumour cell lysis. Besides T cells, other immune cells such as macrophages, monocytes, granulocytes, and natural killer (NK) cells also exert tumour-killing effects. A number of bsAbs including Triomab, BiTE, DART, and FynomAb provide new treatment options for patients.
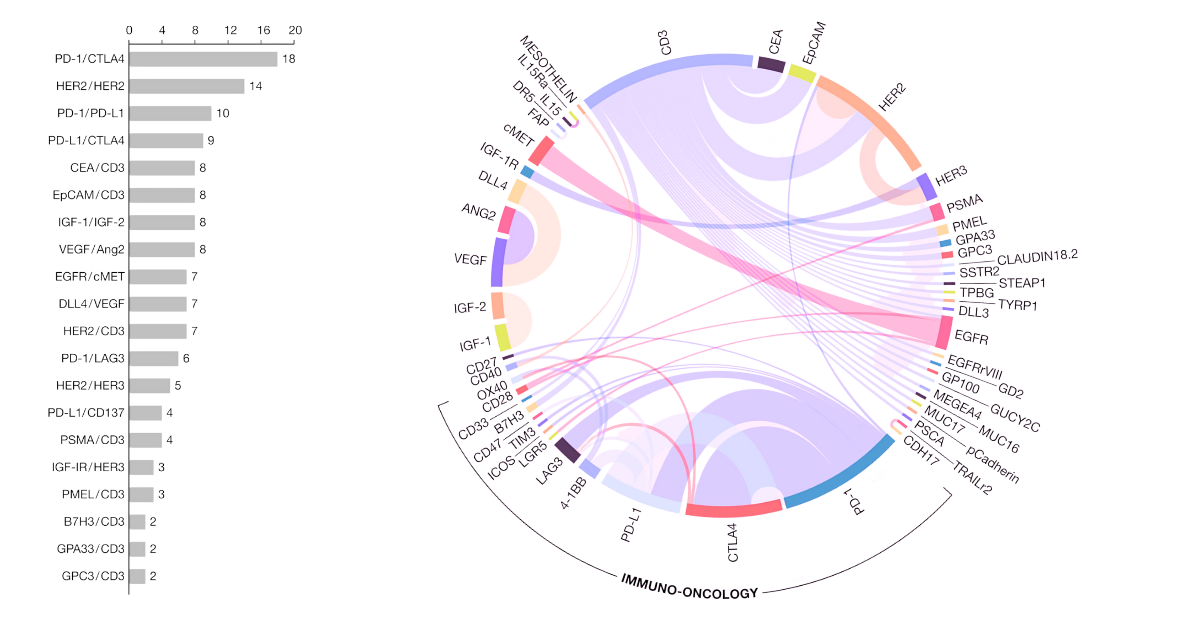
Target combinations for solid tumour therapy. EMBO Mol Med, Volume: 13, Issue: 9, First published: 24 August 2021, DOI: (10.15252/emmm.202114291)
The first bispecific antibody to gain regulatory approval, blinatumomab, targets CD19 on B cells and CD3 on T cells, leading to the activation of T cells and the destruction of B cells. This approach has been successful in the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia (B-ALL). Other bispecific antibodies targeting CD3, such as mosunetuzumab, solitomab and REGN1979, are currently in clinical development for various types of lymphoma. Additionally, several BiTE molecules (targeting CD3 and a tumour antigen) are already in clinical trials for oncology.
CD3:
| CD3 epsilon | BCMA | DLL3 | CD19 | PSMA |
| CD20 | EpCAM | CEACAM-5 | IL-13R | CD4 |
| MUC16 | CD22 | CD38 | ROR1 | Epha2 |
| EFGR | CD28 | CD3E delta | CD133 | CD123 |
| PD-1 | PD-L1 | HER2 | TROP2 | 5T4 |
| B7-H3 | CD79B | PSCA | GPA33 | MUC-1 |
A promising application of bsAbs is to cross the blood-brain barrier (BBB) to target pathogenesis mediators in neurological diseases. The BBB forms a forbidden zone for monospecific antibody therapy. Several bispecific antibodies have been developed to target 4-1BB, including PF-05082566, utomilumab, and mosunetuzumab. These bispecific antibodies are designed to target both 4-1BB and a tumour-specific antigen, leading to the activation and expansion of tumour-specific T cells. It has also been studied in conjunction with PD-1. Early clinical studies of these bispecific antibodies have shown promising results in various types of solid tumours and haematological malignancies. However, 4-1BB presents far more promise than simply onco-therapies with neuro-therapies and angiogenic development when targeting a CNS target.
4-1BB
HER2
CD47
Angiogenesis is a key process in tumour growth and metastasis. Multiple angiogenic factors including endothelial growth factor receptor 2 (VEGFR2), VEGFR3, endothelial growth factor A (VEGFA), angiopoietins, and platelet-derived growth factors (PDGFs) are involved in tumour angiogenesis. Many cancer therapies disrupt angiogenesis by depleting these proteins. Dual targeting of angiogenic factors leads to superior outcomes.
VEGF
Acquisition of resistance to targeted therapy mainly involves the activation of intracellular signals through homologous or heterologous dimers between RTKs and the activation of compensatory signalling pathways. To solve the problem of drug resistance, researchers and clinicians are constantly searching for new targets, developing new-generation targeted drugs and combinatorial treatments. In lung cancer specifically, for example, bsAbs are designed to target the ErbB RTK family members, which comprise EGFR, HER2, HER3, and human epidermal growth factor receptor-4, and participate in overlapping downstream signalling pathways.
EGFR
The blockade of immune checkpoints, such as PD-1/PD-L1 and CTLA-4, has revolutionised cancer treatment. Bispecific antibodies targeting both a checkpoint inhibitor and a tumour-specific antigen have the potential to further enhance anti-tumour immunity. Several such bispecific antibodies, including tebotelimab and IBI939, are currently in clinical development. Recent studies and trends have also shown an increase in the development of bsAbs that are dual immune checkpoint inhibitors, or immune checkpoint/suppressor antibodies.
PD-1
PD-L1
CTLA-4
Cytokines targeting two binding sites can modulate the immune response and potentially treat autoimmune diseases. For example, AMG 592 targets both IL-23 and IL-17, two cytokines involved in the pathogenesis of psoriasis. Other bispecific antibodies targeting cytokines, such as TNF-alpha and IL-6, are also being developed for various indications.
Emerging targets:
Another interesting application of bsAbs is the delivery of payloads such as drugs, radiolabels, and nanoparticles. The payloads are administered once the unbound bispecific molecules are cleared from the bloodstream. Bispecific molecules can be used to enrich payloads in tumour sites. This strategy significantly prolongs the serum retention time and improves the tumour/blood ratio.
The distinct pharmacokinetics of BsAb, however, requires them to be used in a different way than MAbs. Adoptive cellular therapy, using effector cells ‘armed’ with BsAb, might significantly improve the therapeutic utility of BsAb and an increased understanding of the underlying mechanisms of successful antibody-based therapies will allow better strategies to be developed. Since BsAbs can not only bridge therapeutics (e.g., T cells, drugs) and targets (e.g., tumours) but also simultaneously block two different pathogenic mediators, they are posed to improve treatment options against cancer, autoimmune diseases, and inflammatory diseases.
References:
Fan G, Wang Z, Hao M, Li J. Bispecific antibodies and their applications. J Hematol Oncol. 2015 Dec 21;8:130. doi: 10.1186/s13045-015-0227-0. PMID: 26692321; PMCID: PMC4687327.
Zugmaier G, Klinger M, Schmidt M, Subklewe M. Clinical overview of anti-CD19 BiTE® and ex vivo data from anti-CD33 BiTE® as examples for retargeting T cells in hematologic malignancies. Mol Immunol. 2015;67(2):58–66.
Stanimirovic D, Kemmerich K, Haqqani AS, Farrington GK: Engineering and pharmacology of blood–brain barrier-permeable bispecific antibodies. Adv Pharmacol. 2014;71:301-335.
Bastiaan L. Duivelshof, Alain Beck, Davy Guillarme, Valentina D’Atri, Bispecific antibody characterization by a combination of intact and site-specific/chain-specific LC/MS techniques, Talanta, Volume 236, 2022, 122836.
Wang W, Qiu T, Li F, Ren S. Current status and future perspectives of bispecific antibodies in the treatment of lung cancer. Chinese Medical Journal. 2023 Feb. DOI: 10.1097/cm9.0000000000002460. PMID: 36848213.
Krishgen offers ELISA for all of the above targets, as well as a range of (CiteAb 2023 Innovation Award Highly Commended) ELISA for monitoring and quantifying Bispecific Antibody Drugs.
Originally posted by Krishgen Biosystems on https://www.krishgen.com/blog/details/popular-targets-bispecific-antibody-drugs
Caltag Medsystems is the distributor of Krishgen Biosystems’ products in the UK and Ireland. If you have any questions about these products, please contact us.
