Without stabilisation, flow cytometric analysis of blood samples must be performed within 48 hours of venepuncture, as apoptosis and proteolytic degradation of cellular markers will detrimentally affect the immunophenotypic profile. Blood samples older than 48 hours are often no longer suitable for flow cytometric examination, exhibiting indistinguishable cell subsets and inaccurate absolute cell counts leading to erroneous clinical results. This may require a fresh sample to be taken, incurring extra costs and delayed analysis.
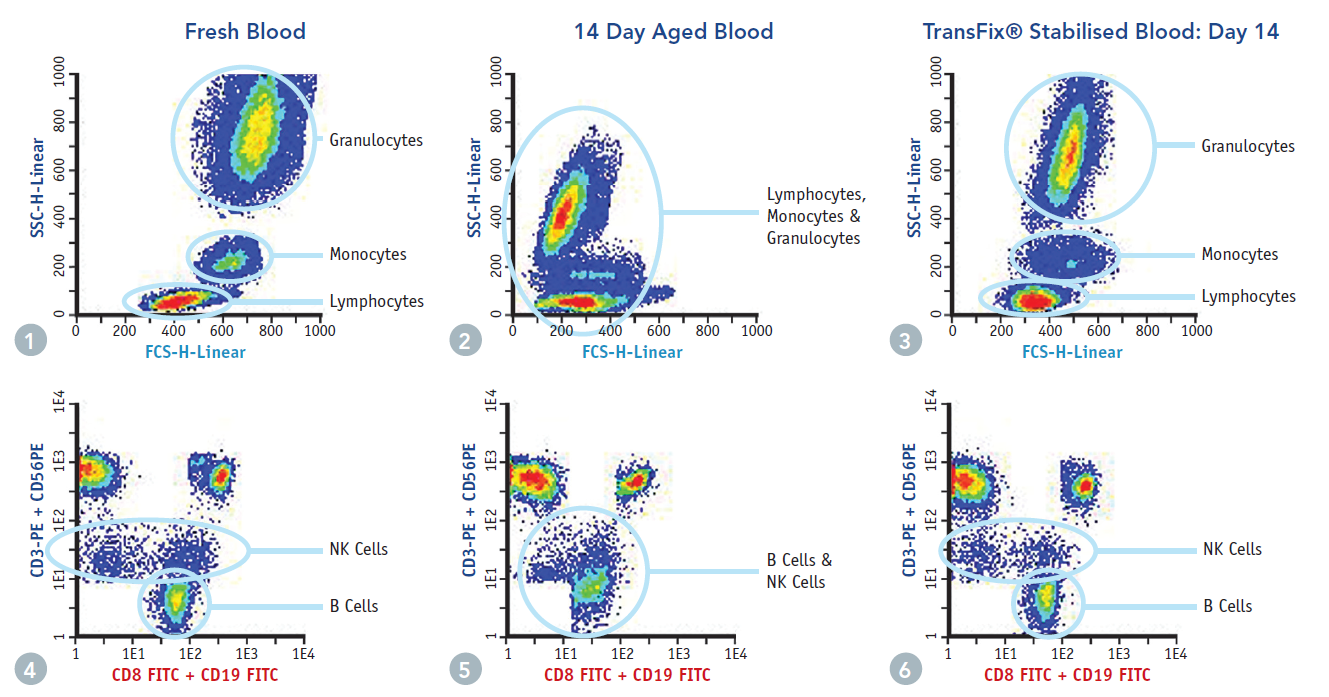 TransFix is a solution that stabilises leucocytes and leucocytic antigens for up to 14 days, preserving the absolute cell counts found in fresh blood and allowing differentiation of leucocyte sub-populations. This allows for an extended time frame for analytical testing, particularly useful for samples coming in late on a Friday afternoon, or for samples which require transport before analysis.
TransFix is a solution that stabilises leucocytes and leucocytic antigens for up to 14 days, preserving the absolute cell counts found in fresh blood and allowing differentiation of leucocyte sub-populations. This allows for an extended time frame for analytical testing, particularly useful for samples coming in late on a Friday afternoon, or for samples which require transport before analysis.
Jump to the relevant section below:
- Advantages for Routine Use
- Advantages For Clinical Research
- Preparing Blood Controls for Immunophenotyping
- Products for Stabilising Blood
Advantages for Routine Use
Immunophenotyping and diagnosis of haematological malignancies (e.g. leukaemia) by flow cytometry
Leukaemia is a malignant progressive disease where bone marrow and other blood-forming organs produce increased numbers of immature or abnormal leucocytes.
Each class of leucocytes express a unique combination of antigens on the cell surface (CD markers). A pathological cell may express more or less of a particular marker than a healthy cell, or additional CD markers.
It is important for clinicians to know what markers are expressed on pathological cells to get an accurate diagnosis. The time taken to perform ‘primary analysis’, (i.e. the initial screen) means that the sample is often not fresh enough to undergo detailed phenotyping at a later date. By stabilising samples at the point of collection, the immunophenotype at this time point is preserved.
See Case Study 1, 2, 3 on the Cytomark website for published examples
Immune Monitoring of HIV/AIDS Patients
Monitoring CD4 and CD8 T-cell subsets of HIV-infected individuals is performed routinely by flow cytometry. As the disease progresses, the number of CD4+ cells reduce, destroyed by CD8+ T cells. Ordinarily, specimens are processed within 36 hours to obtain reliable CD4+ T-cell absolute counts.
By stabilising human whole blood, samples can be shipped at ambient temperature for up to 4 days. This is particularly important in countries where clinics are remote and the transport infrastructure is unreliable.
See Case Study 4, 5, 6 on the Cytomark website for published examples
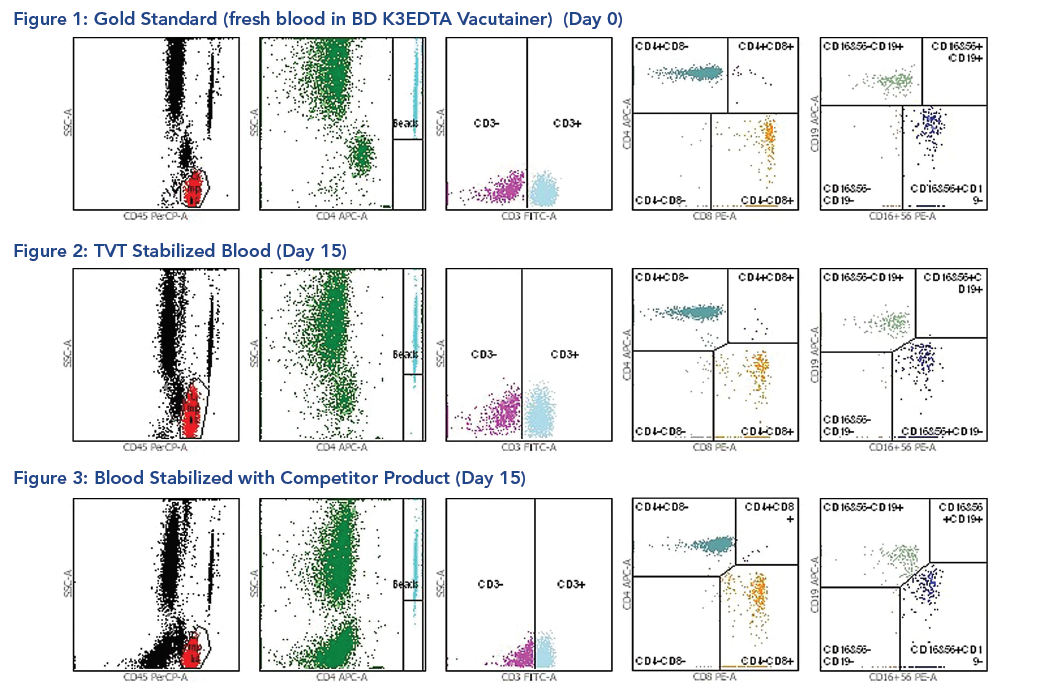
An independent study has also shown that TransFix is a highly effective anti-viral agent that results in a reduction in HIV replication as measured by HIV p24 antigen production (see Case Study 7).
Benefits of Stabilising Whole Blood for Immunophenotyping
- Flexibility in Analysis
- Eliminates out of hours work
- Ability to batch samples
- Reduces impact of unexpected machine breakdown and staff shortages.
- Ability to Ship Samples
- Samples can be shipped over long distances to reference laboratories without requiring cold chain transportation which provides significant cost savings
- Large batches of samples can be shipped together, reducing the demand to ship frequently to centralised laboratories.
- Reduction in False Negative Results (aberrant cell populations are detected)
- Fewer patient recalls due to unusable sample
- Earlier diagnosis and treatment
- Time saved to analyse more samples
- Enable Repeat Analysis without Patient Recall
- Further tests can be performed after the initial analysis if there was an abnormal result
- Eliminate the need for patient recall speeds up diagnosis.
Advantages For Clinical Research
Sample Storage and Transportation
TransFix provides a major benefit for clinical research , especially in the case of multicentre studies, where samples are routinely transported between collection sites and an analytical laboratory.
TransFix stabilised samples can be stored and transported at ambient temperature (18-25°C), removing the requirement for cold chain transportation, providing significant cost reduction (1).
Many clinical trials will collect samples from different sites. TransFix allows samples to be batched and shipped to a single testing facility thus reducing inter-site variability. In addition, shipping costs are reduced as samples from each site can be batched and sent as a consolidated shipment.
Clinical trials often require the collection of clinical samples over multiple time points. TransFix can be used to preserve a snapshot of the immune profile at each time point, plus samples can be batched and processed together.
See Case Study 8 on the Cytomark website for published example of the use of TransFix phase I clinical trial.
Benefits of TransFix for Clinical Research:
- Improve data quality over transit, where immediate analysis is not possible.
- Standardisation of samples across multiple sites (all samples preserved at the same time point, all analysis can be performed at the same site).
- Batch samples for high-throughput analysis.
- Financial savings: Expensive transportation (cold chain storage) is not required.
- Single tube pack sizes available.
Cytomark provide a TransFix Phlebotomy Pack, which provides an individually wrapped TransFix/EDTA Vacuum Blood Collection Tube, with a safety holder with integrated and orientated needle. These are available in multiple needle sizes and draw volumes, and are designed for ease of use in collecting TransFix-stabilised human blood by CROs during clinical trials. Click here for further details.
Preparing Blood Controls for Immunophenotyping
Towards Meeting the ISO15189:2012 Standard in the Clinical Flow Laboratory
The ISO standard entitled ‘ISO 15189: Medical laboratories – Particular requirements for quality and competence’, refers to the ‘use of quality control materials that react to the examining system in a manner as close as possible to patient samples’ (section 5.6.2.2), and that ‘the use of independent third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer ‘(section 5.6.2.2, Note 2).
It is common practice in immunophenotyping and immune monitoring laboratories to use a patient sample as a daily or even weekly control for flow cytometry. In addition, when a rare immunophenotype is detected there is an opportunity to preserve it for teaching or comparison purposes.
All samples left untreated are subject to apoptosis of cells and proteolytic degradation leading to immediate changes in the phenotypic profile. This poses a challenge, not only with analysing the sample in a short time scale but when preserving samples of interest for re-testing.
Simple addition of TransFix to a blood sample enables stabilisation of the immunophenotype for up to 14 days. Subject to validation at a clinical flow facility, this could provide a solution when such reference material is not available and would address the clauses referenced above.
Therefore, using TransFix, it is possible to stabilise blood samples (diseased and healthy) for use as a reference tool during flow cytometry analysis.
Protocol – Preparing a Blood Control
- Collect blood by venepuncture into an EDTA vacuum tube according to CLSI document H3-A62.
- Carefully remove the blood collection tube cap and determine the volume of anti-coagulated whole blood within the vacuum tube.
- Pipette into the blood collection tube the appropriate volume of TransFix at the ratio of 0.2ml TransFix per 1ml of blood. For TransFix Sample Storage Tubes, add 1ml blood to the tube. Note: Blood samples should be treated with TransFix immediately after collection, but failing this, blood must be less than 6 hours old when it is treated with TransFix. Do not refrigerate the sample before treatment with TransFix.
- Replace the cap on the blood collection tube, ensuring that there is no leakage and mix gently by inversion at least 10 times. Inadequate or delayed mixing may result in inaccurate test results. Do not vortex.
- Once TransFix treated, the stabilised sample can be transferred to smaller test tubes to prevent repetitive cooling and warming thereby preserving the phenotypic profile of the sample.
- Store/transport the TransFix treated blood for up to 14 days at 2 – 8°C or for up to 4 days at 18 – 25°C.
- If refrigerated, incubate the treated blood sample at room temperature (18 – 25°C) for 15 minutes prior to use. Then mix the treated blood by rolling the tube between the hands 10 times and by inverting. Take care when opening blood collection tubes and briefly spin TransFix Sample Storage Tubes to reduce the build-up of blood in the caps.
- Perform analysis by flow cytometry in accordance with the manufacturer’s instructions. A ‘stain, lyse no wash’ sample preparation method is recommended. Blood stabilised by TransFix should be analysed within 6 hours before being returned to 2 – 8°C storage for future use, if necessary.
Note a: Light scatter positions of cells stabilised by TransFix may differ slightly from those of untreated cells.
Note b: The dilution factor must be accounted for when calculating absolute cell counts. This can be done by multiplying the value given by the manufacturer to the absolute counting beads by 1.2 so that absolute cell counts are automatically corrected for TransFix treated blood samples.
Note c: Studies have shown that moderately high levels of haemolysis, icterus and lipemia do not affect the results. Grossly haemolysed samples should be rejected.
Products for Stabilising Blood
 TransFix Products are sold globally under the Cytomark brand name. Customers in the UK and Ireland can purchase from Caltag Medsystems Limited:
TransFix Products are sold globally under the Cytomark brand name. Customers in the UK and Ireland can purchase from Caltag Medsystems Limited:
- Bergeron et al. (2002). Evaluation of stabilised blood cell products as candidate preparations for quality assessment programs for CD4 T-cell counting. Clinical Cytometry 50, 86-91
