Contents
- History
- Principle
- How Does It Work?
- What Are The Applications?
- What Is The Protocol?
- What are the main differences between Lipofection and Polyfection?
A New Transfections Reagent, Not Just Another One
After the development of Lipofection (lipid-based transfection method) and MagnetofectionTM (magnetic nanoparticles-based transfection method), OZ Biosciences revolutionises Polyfection with the design and synthesis of a novel patented Cationic Hydroxylated Amphipilic Multi-block Polymer (CHAMP) which is biocompatible, cleavable, ph responsive and bi-functional.
OZ Biosciences created a new transfection agent based on the CHAMP technology to mark the separation from what is usually being done with classic transfection methods. This novel bi-functional copolymer is biocompatible, ionisable and pH sensitive. Formed by three moieties, it combines and introduces three synergistic notions:
- The concept of “passing through the membrane barriers” due to its charge, pH-sensitive and hydrophobic properties.
- The idea of “stealth transfection”, where DNA is protected, masked and supported all the way to its nuclear uptake.
- The concept of biocompatibility, due to biodegradable and cleavable moieties.
This polymer-based transfection technology is an optimised delivery system that allows high efficiency with low cellular stress thanks to improved delivery mechanisms.
History
Two main types of delivery vehicles are routinely used for genetic modification of cells: viral and non-viral vectors.
Transport systems have to overcome a series of extracellular and intracellular barriers until the DNA is delivered into the cell nucleus. Viruses have evolved in order to bypass each of these checkpoints but despite their efficiency, they have to deal with important issues such as immunogenicity, cytotoxicity, safety and target-cell specificity that limit their use.
Inspired by the strategy of some viruses to gain entry into mammalian cells, researchers tried to build synthetic viruses or virus-like particles in order to efficiently and safely transport genetic material to the cell nucleus; the main focus being to mimic viral vectors in terms of performance without encountering their principal pitfalls[i]. The goal was to replicate with the synthetic molecular complexes all the steps used by the viruses to infect mammalian cells.
Non-viral vectors have thus gained increasing attention for several decades as they do not contain any pathogenic proteins and are therefore more likely to be safe[ii]. However, synthetic carriers were generally unsatisfactory because they lacked one or several of the necessary functions needed for optimal performance.
Synthetic vectors are materials that electrostatically bind and compact nucleic acid into nanoparticles (tens to several hundreds of nm), protect them from degradation and mediate their entry into cells. Cationic lipids and polymers can be used to complex DNA, creating lipoplexes and polyplexes respectively. The use of cationic lipids for gene delivery was first reported by Felgner in 1987 [iii] and lipofection mechanisms are described elsewhere ([iv]).
Synthetic polymers were also extensively studied principally due to their chemical versatility that “easily” allows generating, modifying and synthesising linear, branched or dendritic polymeric structures with multiple functions[v].
Cationic polymers play a crucial role in the development of gene transfer agents due to their extraordinarily good potential to condense DNA [vi]. As a result, cationic polymers hold great promise for gene delivery and one of the most used polymers for gene delivery is polyethylenimine (PEI)[vii]. Numerous drawbacks of PEI have limited its application and many alternatives (polylysine, polyamidoamine, dendrimer, polyallylamine and methacrylate/methacrylamide polymers) have been synthesised gaining ground on efficacy and reducing toxicity without however reaching all the promises.
One of the main issues remains the activation of innate immune response induced by the gene delivery system[viii]; trying too hard to mimic virus physiology can result in reaching the dark side. Gene delivery is sensed as a viral or bacterial attack by the cell that answers by disrupting foreign nucleic acid, inactivating transgene expression or undergoing apoptosis. The overall efficiency is thus lowered.
Up to now, no real breakthrough has emerged in the cationic polymer field. Helix-IN DNA transfection reagent, OZ Biosciences’ new CHAMP polymer, presents all the characteristics of a classic cationic polymer enhanced with a nucleic acid shielding core to lower cellular stress and combined with a biodegradable property; which leads to an enhanced transfection.
Principle
BI-FUNCTIONAL DUAL COPOLYMER STRUCTURE: DNA PACKAGING
The particularity of this novel CHAMP technology comes from the fact that the bi-functional cationic biopolymer is made up of three moieties, each bearing different characteristics and functions.
- The first part in the vicinity of the polymer binds and condenses DNA to an unprecedented level and contributes to cytosol delivery.
- The second component is a pH-responsive and cleavable linker that improves cellular delivery by favouring endosomal membrane destabilisation.
- The third moiety with a defined and optimised molecular weight serves as a DNA shield and nuclear uptake facilitator.
The molecular weight and length (unique for each type of polymer) of each moiety are important parameters linked to overall transfection efficiency.
How Does It Work?
1. Protection and Serum Stability
The design of Helix-IN allows: (1) positively charged polyplexes to be stable in solution and (2) not to aggregate over time.
The structure, polyamine composition & grafting density of the CHAMP polymers were finely tuned and optimised to place the polyplexes at the exact interface where solubility is not affected over time. Moreover, hydrophilic groups were ingeniously arranged within the polymer to lower interactions with negatively charged serum proteins (albumin…) for a more efficient gene carrier definition.
Polyplexes remain intact and DNA is protected from degradation…
This positively charged bi-functional polymer presents enhanced DNA-binding properties allowing extent protection of DNA; the positive DNA/polymer charge ratio keeps DNA bound to polymer, playing a key role in protecting nucleic acid from degradation by serum enzyme. OZ Biosciences designed this polymer so that no DNA degradation is observed even when incubated in 50% fetal calf serum at 37°C for 24H.
2. Cellular Uptake
Cationic complexes bind to cell membranes mainly through electrostatic interactions (figure 1 – 1) and most polyplexes are taken up by the cell through endocytosis pathways (macropinocytosis, phagocytosis, endocytosis). One of the most documented routes of endocytosis is mediated by clathrin (figure 1 – 2). Once endocytosed, complexes are internalised in an early endosome where pH drops from 7.4 (cell surface) to 6.0 (lumen of endosome). The pH will drop to 5 as the endosome progresses to its late phase.
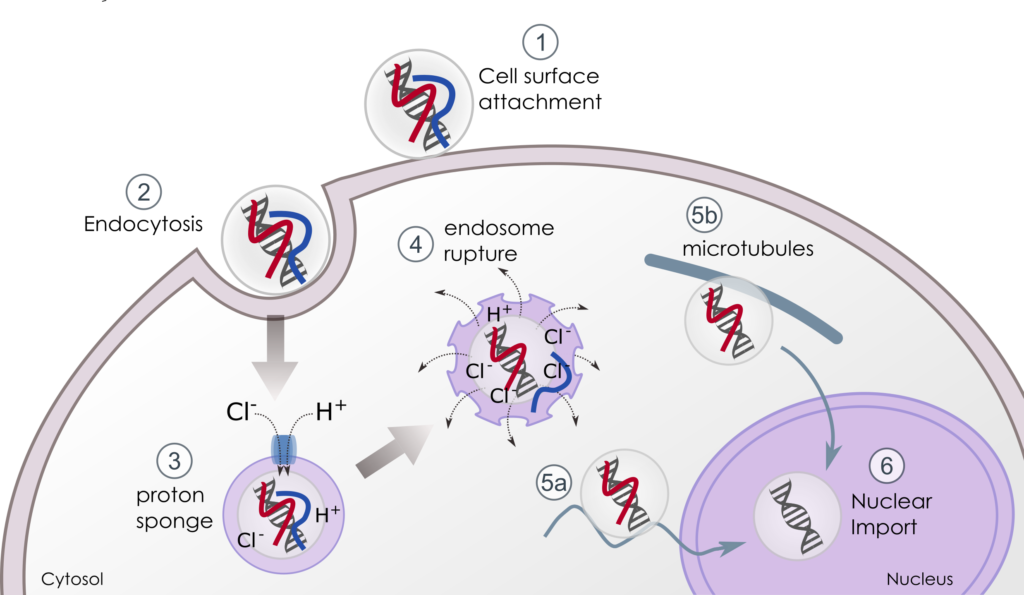
3. Endosomal Escape & DNA Release
Polyplexes evade endosomes and release their cargo into the nucleus through the cationic polymer buffering capacities related to the “proton sponge” effect (ix).
The protonable amines acting as a weak base in an acidic medium destabilises the pH inside the endosome: once inside the endosomes, specific ATPases generate an influx of protons that are buffered by the polymer (figure 1 – 3). The massive and continuous flow of protons is accompanied by the passive entry of chloride ions that results in the accumulation of water. As a consequence, the vesicles swell until endosomal rupture and their content is delivered into the cytosol (figure 1 – 4).
The first polymeric block plays this role. Moreover the pH responsive and cleavable hydrophobic part adds supplementary features. Indeed, the linker hidden at physiological pH gets exposed at acidic pH. This leads to its cleavage and to the hydrophobic zone exposition which promotes endosomal membrane fusion/destabilisation.
At this stage, several important pitfalls can impair transfection efficiency:
- The capacity of DNA to escape from endosomes is one of the major limitations of transfection.
- It is generally admitted that once delivered into the cytosol, DNA must rapidly be imported into the nucleus to avoid cytosolic degradation.
- The presence of cell sensors in endosomes (also on the cell surface) that can recognise foreign nucleic acids and induce a protective response inhibiting transfection
Bi-functional co-polymer: inside the cell, the first block that compacts the DNA presents high buffering capacities that favour endosomal swelling, the pH-responsive cleavable linker promotes membrane destabilisation and releases stealth DNA into the cytosol; the third block protects and guides DNA into the nucleus.
4. Transport & Nuclear Internalisation
Once released from endosomes, polyplexes have to migrate to the nucleus either via microtubules (figure 1 – 5a) or through nuclear import machinery (figure 1 – 5b). In general, large DNA molecules (>3000bp) and polyplexes remain almost immobile as diffusion is size-dependent into the cytoplasm[ii] and numerous cytosolic nucleases degrade nucleic acids. Being still complexed to the third moiety of our bi-functional polymer, the smaller positively charged polyplexes can interact with anionic microtubules or motor proteins, or diffuse in a stealth mode until their nuclear uptake. During all these procedures, the DNA is masked and protected from degradation.
The most evident way of nuclear entry for immortalised cells is during mitosis where redistribution of cellular material occurs and the nuclear membrane is disrupted, however, not all the cells follow a proliferative pattern. Up to now, little is known about the nuclear import of polyplex vectors. As soon as DNA has reached the nucleus, it is released from the second part of the polyplexes whose positive charges, molecular mass and grafting design were designed to improve transfection.
What Are The Applications?
Helix-IN™ DNA Transfection Reagent
The principal use is DNA transfection for in vitro and in vivo applications.
The CHAMP technology increases transfection: more DNA enters the cells and DNA is addressed to the nucleus in a stealth mode without alerting and stressing the cells. This reagent is ideal for immortalised cell lines preferentially adherent such as HEK-293, NIH-3T3, CHO, COS, HeLa, MCF7, MEF, RPE-1; C2C12, etc.
It is perfect for co-transfection of multiple DNA.
In vivo, DNA is condensed and protected into small polyplexes that limit immune responses and are able to navigate through the circulatory system until they deliver.
What Is The Protocol?
The protocol is simple: transfection reagent is directly mixed with DNA using ratios 1:1 to 3:1 (1µL per µg DNA to 3µL per µg DNA) depending on the cell type. After 30 min of incubation time, polyplexes and boost are added into cells.
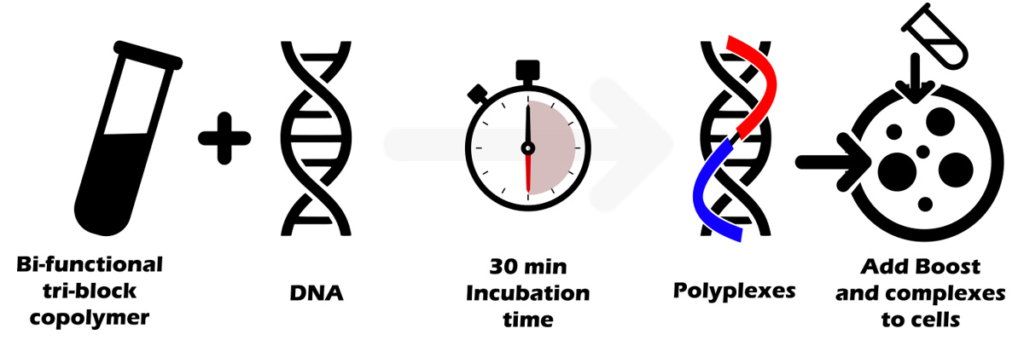
This 30 minute incubation time is the cornerstone of the protocol allowing a full compaction and protection of DNA.
During the nanoparticles/DNA complexes self-assembly, it is critical to wait at least 30 minutes to enable the co-polymers and DNA to form stable supramolecular nanoparticles. Due to the multipart nature of the copolymer, the time for forming and stabilising the complexes is slightly longer than with “simple” polymers where the formation of complexes occurs more rapidly (10-20 min).
What are the main differences between Lipofection and Polyfection?
Lipofection and Polyfection (respectively lipid-based and polymer-based transfection) are two methods of transfection using synthetic vectors to deliver nucleic acids into cells. Even if the finalities of the two techniques are the same, some differences still exist orienting the nucleic acid delivery applications to one or the other (refer to the table below).
The principal difference between the two technologies comes from the capacity to “easily” modify, graft, branch or change the amount of amines or hydrophobicity of polymers; this results in highly condensing and compacting DNA for more efficient delivery into the nucleus. Lipofection’s main advantage relies on its versatility: all nucleic acids and even proteins can be delivered into cells as opposed to Polyfection, which is preferentially dedicated to DNA applications.
The choice of the method for transfection will depend on the cell type and the application. Lipid-based reagents are less recommended if lipid signalling is studied when Polyfection will not be advised for transfecting suspension cell lines. Both techniques are compatible with stable cell line generation and should be approached sparingly when dealing with primary cells: in this case, Magnetofection must be highly considered.
| Lipofection | Polyfection | |
|---|---|---|
| Structure, properties, mechanism delivery | 1. Liposomes, micelles, inversed micelles (amphipathic) – hydrophobic 2. Fusion/destabilisation/flip-flop 3. Cytoplasmic release 4. Can be used alone or in presence of co-lipid 5. Generally based on the same model: hydrophilic head group, hydrophobic anchor and linker | 1. Linear, branched or spherical 2. Water soluble, high charge density 3. Proton-sponge effect 4. Nuclear uptake possible 5. Various designs, grafting composition, length, MW… |
| Strengths | Versatile: any nucleic acids Biodegradable Excellent biocompatibility with cellular membrane No package size limits Short time required for formation of complexes Superior bioavailability when complexes are formed | High DNA condensation/delivery Biodegradable Low cellular stress High structural integrity and stability over storage Low toxicity (low to medium MW polymers) Stealth transfection Increased stability of polyplexes over time No Autofluorescence |
| Weaknesses | Autofluorescence Low structural integrity Can interfere with lipids signaling Fast clearance in vivo in systemic circulation Mild inflammatory response in vivo Not applicable to all cells (primary) Need of a colipid | Not applicable to all nucleic acids Not good for suspension cells HMW polymers show toxicity. Not applicable to all cells (primary). |
References
[i] Slivac, I. et al (2016). Non-viral nucleic acid delivery methods. Exp. Op. Biol Ther. Vol 17, 2017 – Issue 1
[ii] Xiang, Y. et al (2017). Recent development of synthetic nonviral systems for sustained gene delivery. Drug Discov Today. 22(9):1318-1335.
[iii] Felgner, PL. et al (1987). Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 84(21):7413-7.
[iv] Cullis, PR. et al (2017). Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol Ther. 5;25(7):1467-1475.
[v] Prabu, SL. et al (2017). Biopolymer in Gene Delivery. Intech book Chapter 7. DOI: 10.5772/65694
[vi] Ruponen, M. et al (1999). Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: Physicochemical and transfection studies. Biochimica et Biophysica Acta: Biomembranes, vol. 1415, no. 2, pp. 331–341.
[vii] Boussif, O. et al. (1995). A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7297-301.
[viii] Sakurai, H. et al. (2005). Innate immune response induced by gene delivery vectors. Int J Pharm. 354(1-2):9-15.
[ix] Kim, TI. Et al. (2011). Bioreducible polymers with cell penetrating and endosome buffering functionality for gene delivery systems. J Control Release. 152(1):110-9.
[x] Luby-Phelps K (1987). Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc Natl Acad Sci U S A. 84(14):4910-3.
Originally posted by OZ Biosciences on: https://ozbiosciences.com/content/10-the-champ-technology
Caltag Medsystems is the distributor of OZ Biosciences products in the UK and Ireland. If you have any questions about these products, please contact us.
